Can exposure to 670nm infra-red light improve retinal function and structure?
BUS is delighted to announce forthcoming research into a novel treatment for birdshot which is being trialled at Moorfields Eyes Hospital. The Director of Medical Retina and Uveitis, consultant Carlos Pavesio, the lead researcher, will be investigating whether using a particular wavelength of light will reduce inflammation in the eye and improve retinal function and structure in birdshot uveitis.
Please note, that before everybody rushes forward to volunteer, the patients selected from Carlos Pavesio’s clinic will be invited to participate in this research only if they fulfil the necessary criteria, and initially, only 10 to 12 patients will be required. His research team aims to study the effects of 670nm infra-red light treatment on patients with ‘grumbling’ birdshot disease, with their signs of inflammation continuing to be treated with medication throughout the trial.
Funding for the project
Half of the money for this research proposal was raised by birdshot walkers on the 2012 Fight for Sight ‘Carrots Night Walk’. We are collaborating with Fight for Sight, the main UK charity dedicated to funding eye research to prevent sight loss and treat eye disease, who have matched our fundraising to help pay for this novel piece of research via their jointly funded Small Grant Awards programme.
Targeted treatment
Birdshot is an eye disease with no known involvement of other parts of the body. Because of this, effective treatment directed just to the eyes would be a big step forward.
670nm infra-red is a part of the natural light spectrum. The energy levels and wavelength of the 670nm light used in the study are lower than those of indirect light on a spring morning. This light wavelength is also emitted from conventional light bulbs, but it is absent from strip lights and also absent from all the new low energy eco-friendly domestic light bulbs.
Study patients will receive 670nm light treatment from a specially-designed light. Treatments will be given on weekdays for two weeks for a total of ten treatments. Each light treatment lasts one minute. Only one eye per patient will be treated but the other eye will be observed as a ‘control’ for comparison.
In active birdshot uveitis, there is a measurable delay in response to the 30Hz ERG flicker test stimulus, so before the first light treatment for each patient in the trial, a baseline comprehensive ERG will be done. During the trial, patients will be given further ERGs before and after each light treatment, and these ERGs will be performed with a hand-held ERG device (RETeval) where the electrodes are placed on the skin under the eye. These pre- and post-treatment ERGs will check if the light treatment has improved the ERG response.
It will take no more than two minutes to do the study ERG tests, and the eyes do not have to be dilated. The ERG results will be immediately available, so the clinicians will quickly know if there are any improvements in the eyes.
Depending on the results of this small trial, the researchers aim to run a larger study over a longer period of time to assess the usefulness of this special light therapy as a treatment option for birdshot.
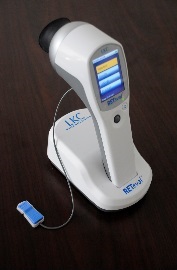 See the Youtube video of the RETeval ERG device by clicking this link
See the Youtube video of the RETeval ERG device by clicking this link
This project combines the skills of the medical retina department at Moorfields Eye Hospital with the experience of using this therapy in the laboratory environment of the Institute of Ophthalmology. Both places are world leaders in addressing ophthalmic problems.
Here’s to success with this study. We will be very interested to hear how the project progresses.
Annie,
I was most interested to hear of this new treatment to be trialled, saw the youtube video of the device and pleased to see development continues for alternative approaches.
I wish the trial every success and also will be keen to hear what progress is made.
Kind Regards,
Dave
Thanks Dave. We will keep you posted. Best wishes Annie
Trying to find an update on the results of this research.
The good news is the trial has started and should soon complete. It took ages to get it though the ethics committee. Health and safety concerns that the it was absolutely safe. We will publish the results as soon as we have them.
Have you heard anything about the red light treatment experiment results yet? Having a lot of bad side affects from the oral meds, and flares as well.
They have still to do the trial on 3 patients. It’s taking longer than expected as each person on the trial has to attend the hospital, three times a week for three weeks, plus have tests before and after the treatment, plus things like public holidays also make it awkward. Now of course we have the summer holiday coming up. We will publish results as soon as they are known.
Thanks for the update. Trying to be patient, but so badly want a better alternative to these nasty meds.
Are there only 3 patients in the trial, or just 3 more patients that need to try the treatment? Have you heard any preliminary results?
There are only three more patients needed to finish the trial. (I am one of them) and am awaiting the timetable for me to start. I think the other two patients have been identified, so hopefully they will run the trial on all three of us at the same time.
Praying for positive results for you and the other trial participants.
Hi, do you know if the trial has been completed yet? Any word on the results so far?
Hello, sorry I am slow to get back to you on this. The study is nearly completed and the results should be out this year. The last group of people being studied just have to have their final ERG.
Why is it taking so long for the results to come out? Will they be posted on this site?
Good question. I asked the same question recently and had a verbal update, but we need to wait until they publish the results. We will post about the results as soon as we can.
Another year has gone by. Did they scrap the project, or did I just miss seeing the results?
Results are being written up. It did show some improvement, but dependent on time of day it was used and not permanent so you need to keep doing it. It won’t replace meds, but it might help. For example improve colour vision. They are testing on old people now to see if it helps AMD. They reckon that a more powerful light required and for a bit longer time, so really more trials on more people required to find the optimum dose. I’ll post a link to the paper when it is published.
Thanks for the update. I look forward to reviewing the paper, once it’s published.
I have only recently heard about this light therapy treatment for BS which is being used to treat other autoimmune diseases, so I am watching with interest for the outcome to be published. I was diagnosed with Birshot in August 2019 and have suffered horrendous side effects from the steriod treatment. I pray that another treatement is found before too long.