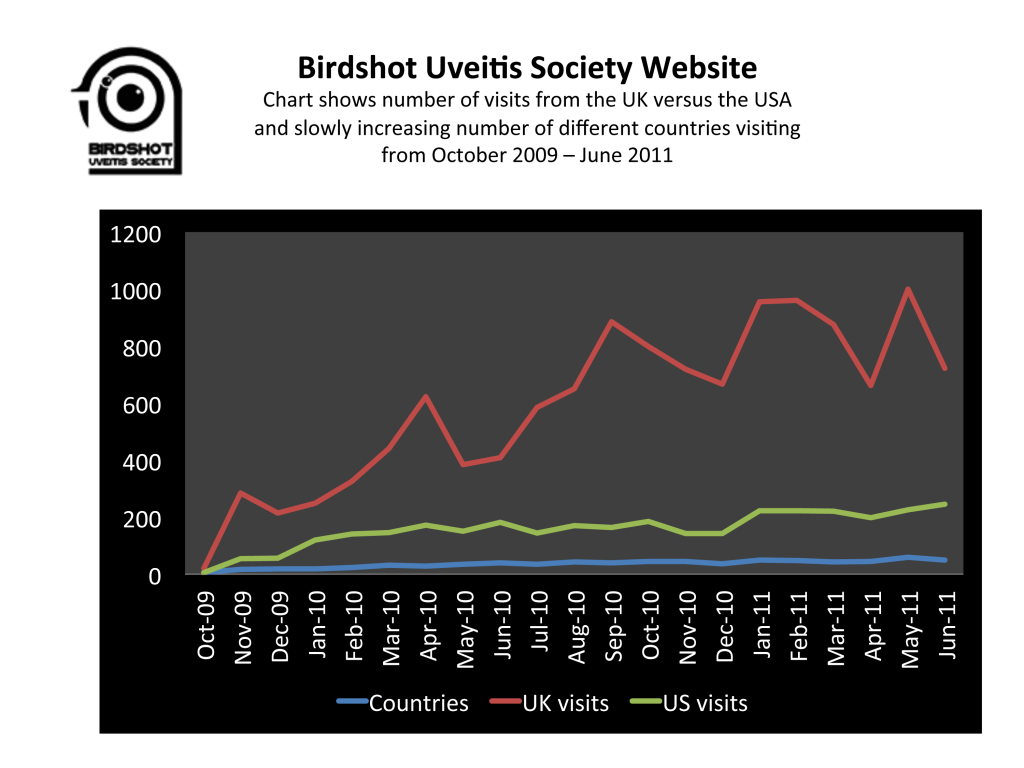
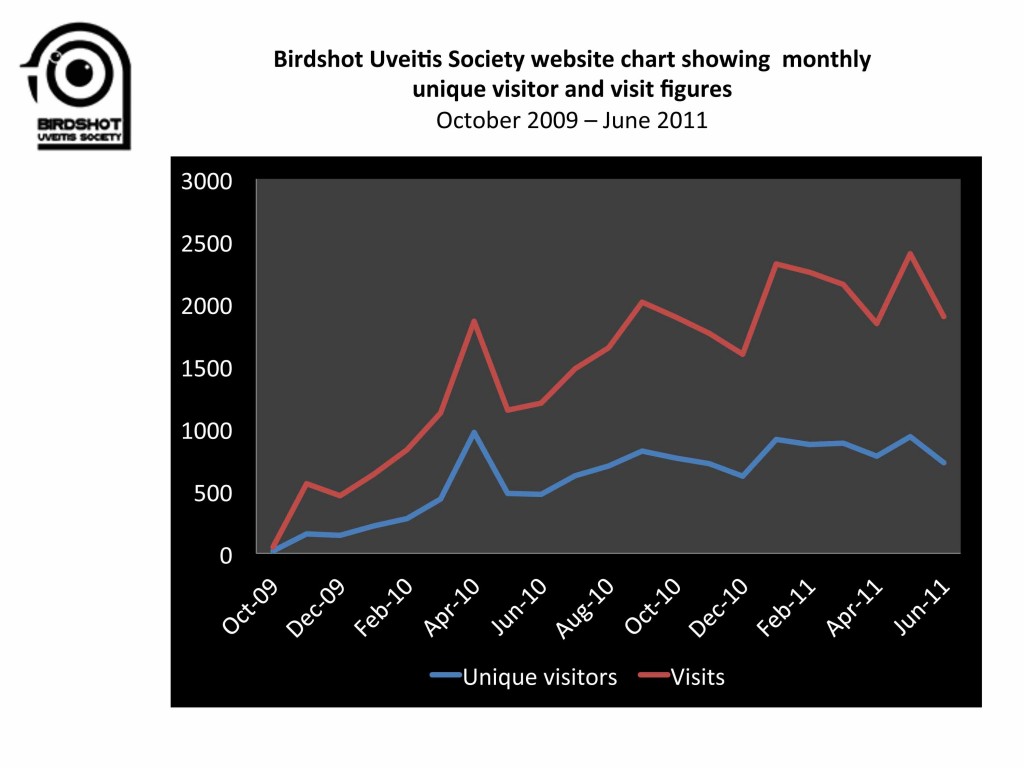
We are re-posting this, as the survey ends on 31 July, and we need as many responses as we can get, in order to make sure that Birdshot becomes a priority for research. If you have not already done so, please complete this survey. It is quick and simple to do. We have posted some ideas about what kind of research is needed into Birdshot at the end of this post, to help stimulate debate, and give you some ideas. Remember that the more people who respond mentioning Birdshot, the more likely we are to get Birdshot as a priority!
The Sight Loss and Vision Survey is a joint initiative between Fight for Sight, The Royal College of Ophthalmologists, The College of Optometrists, the National Institute of Health Research, RNIB and The James Lind Alliance. It has been set up to find those areas of research that have not yet been identified. For us with Birdshot, this is really important, as we have a rare disease, and very little research has been carried out to date. This is our opportunity to get Birdshot (and other rare, auto-immune forms of posterior uveitis) on the national agenda. Because the survey will be completed by patients, it will have great credibility and it is hoped that funds can then be identified for some of the research needs.
The James Lind Alliance is a non-profit making organisation, funded by the National Institute for Health Research, which will oversee this initiative ensuring the exercise produces an unbiased result, with equal weighting being given to each of the participating groups – so your opinion really will count.
The Sight Loss and Vision Survey will allow you to identify your most pressing questions about the prevention, diagnosis and treatment of Birdshot.
The more Birdshotters that complete the survey the more likely we are to be able to influence the research agenda and receive valuable funding from the government for research into Birdshot – we really do need you to take part.
To complete the survey and learn more about this initiative please visit www.sightlosspsp.org.uk where you will find both the online survey and can request alternative formats – post/fax or telephone.
The survey takes less than 10 minutes to complete so please take this opportunity to represent Birdshot and help change the future of eye research.
Thank you!
1. What causes Birdshot?
2. Which part of the immune system becomes disregulated?
3. How do you re-regulate the immune system without damaging the body?
4. How can we find less toxic medication that does not adversely affect mental health and quality of life, but preserves vision?
5. How can we ensure early detection of Birdshot to prevent sight loss?
6. What are the genetic links and why are several generations of some families affected, and why does it seem to affect mainly Caucasians?
7. Why is there a ‘spectrum’ of Birdshot?
8. Why do different people respond differently to different medication regimes?
9. Why is Birdshot treated systemically with toxic medications which adversely affect mental health and quality of life, when it seems to be confined to the eyes?
10.What does the link to HLA A29 mean in relation to treatment?
11. What is the risk/benefit analysis of toxic treatment to prevent blindness versus blindness?
12. What are the long term costs to health and social care of blindness which could have been prevented through the use of off license or off label medications?
13. Is Birdshot best treated by ophthalmologists or should a clinical specialism of immunology be developed?
14. Can holistic therapies such as acupuncture, meditation, hypnotherapy help in preventing or treating Birdshot?
15. Can supplements, such as vitamin D or other therapies treat Birdshot less toxically than current medications?