We are so grateful to Dr Graham Wallace for writing the attached article, which explains the immunological process that leads to Birdshot. Dr Wallace is a member of the Birdshot Research Network and is Senior Lecturer in Immunity and Infection at the University of Birmingham. For those of you who attended the Birdshot Day in March 2012, you will remember that he gave a fascinating talk on ‘The Science of Birdshot’. If you want to see his talk, we have posted it at:
http://www.youtube.com/watch?v=TNGAXyFrm00&feature=plcp
Here is Dr Wallace’s article on ‘An Immunological Basis For Birdshot’
The major histocompatability complex (MHC) is found on chromosome 6 in humans, and contains several genes which are involved in the immune response. Of particular significance are the genes that encode the human leucocyte antigens (HLA) which are fundamental to the immune system. HLA can be separated into two families: class I (A, B and C) and class II (DR and DQ). As class II molecules are not implicated in Birdshot I will not mention them again in the report. Each individual has two copies of HLA-A, B, and C on all cells of the body; one from their mother and one from their father. It is because of this genetic transfer from parent to child, that HLA class I were identified originally as transplantation antigens that must be matched for a successful tissue graft. The reason for this is the function of HLA class I which can be separated into two mechanisms;
(1) HLA class I proteins present small pieces of proteins that have been broken up inside the cell on the surface and these are recognised by immune cells, called cytotoxic CD8 cells, which can kill other cells. This is particularly relevant for protection against viruses which enter human cells and replicate. Some virus proteins are broken down and presented by HLA class I. That virally infected cell can be eliminated and the virus destroyed.
(2) The second function of HLA class I is to protect normal cells from being eliminated by another group of cells called natural killer (NK) cells. NK cells are also cytotoxic but do not recognise protein particles presented by HLA class I, as CD8 cells do. Rather, expression of HLA class I on a cell is recognised by protein called killer immunoglobulin-like receptor (KIR) on the NK cell and this interaction sends a “do not kill” signal to the NK cell and it moves on to check other cells. This benefit of this system is that some viruses cause HLA class I molecules to be removed from the surface of an infected cell thereby preventing recognition of viral fragments by CD8 cells. However such down-regulation of HLA class I will now make these cells susceptible to elimination by NK cells.
As HLA class I is found constitutively on all cells in the body they are not eliminated by NK cells. Bw4 is a region found on several HLA-B molecules.
KIR belong to specific families encoded by genes on chromosome 19 in humans. However, combinations of KIR vary between individuals and different cells may express more than one KIR. Both the inhibiting (KIRL), and activating (KIRDS) can be found on the same cell and it is the complex interaction of these molecules that control NK cells activation. (Fig 1)
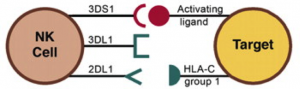
Figure 1 – inhibitory KIRL recognise HLA class I molecules on cells and inhibit killing. Activating ligands induced by challenge are recognised by KIRS molecules. The balance between these signal determines outcome.
The MHC class I molecule, HLA-A*29, is associated with Birdshot uveitis. KIR-HLA pairs implicated for weak inhibition such as KIR3DL1 + HLA-Bw4(T80)) in combination with activating KIR (KIR2DS2, KIR2DS3 and KIR2DS4) were found associated with increased risk in BCR HLA A*29 positive patients with BU. By comparison, association of strong inhibitory pairs such as KIR3DL1+HLA-Bw4(I80) in combination with KIR3DS1 was observed in HLA-A*29-negative controls. These results suggest that a profound effect of activating KIR (KIR2DS2/S3/S4) in the absence of strong inhibition may enhance the activation of natural killer cells and T-cell subsets against intraocular self-antigens, thereby contributing to pathogenesis of BCR.
To confirm the role of HLA-A2902 in the disease the gene inducing the protein was obtained from a patient with Birdshot and used to create a transgenic mouse. These animals did develop ocular disease that was similar to Birdshot, but not until 12 months of age confirming the effect of ageing on the condition.
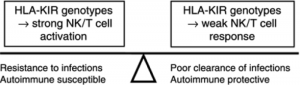
Figure 2 – the reason why certain combinations of HLA and KIR are maintained is because they are useful at protecting against infections, but in combination with other gene polymorphisms this can lead to autoimmune disease.
The challenge in Birdshot as in many other forms of disease is to determine what the effect of the various combinations have at a functional level. ie do cells from patients with certain combinations “kill” less efficiently and what does this mean for the patients. It will also be necessary to analsye other genes for variants that may give an additive effect to the HLA:KIR combination and lead to disease.

 Opticians and optometrists are a group of people BUS really want to reach as they are the normal port of call when something goes wrong with your sight. In my own case I must have gone to different opticians complaining about various symptoms four or five times before one of them realised that there was something wrong with my eyes and asked if I would like to be referred!
Opticians and optometrists are a group of people BUS really want to reach as they are the normal port of call when something goes wrong with your sight. In my own case I must have gone to different opticians complaining about various symptoms four or five times before one of them realised that there was something wrong with my eyes and asked if I would like to be referred! It comes under the Eye Health section. If you type Birdshot into the box it takes you to this link, which in turn provides our website address and the comment:
It comes under the Eye Health section. If you type Birdshot into the box it takes you to this link, which in turn provides our website address and the comment: 














