Birdshot Uveitis Society has noticed that there have been a lot of new development on stem cell research in the news lately which offer new hope to those whose rods and cones are losing their function. Here are a few examples which caught our eye in January and which offer hope for Birdshotters in the future:- Continue reading
BUS News
The National Birdshot Research Network (NBRN)
The National Birdshot Research Network will next meet on Thursday 13th March in London at St Thomas Hospital in the afternoon. Continue reading
Intravitreal bevacizumab (Avastin®) versus triamcinolone (Volon A®)
Birdshotters who have been receiving Avastin® to deal with their macula oedema will no doubt be interested to read the results of this recent study which compares intravitreal bevacizumab (Avastin®) versus the steroid triamcinolone. The trial in question concerned people with diabetic macula oedema. Continue reading
Staying well on immunosuppressive therapy
We are often approached by people who are clearly worried about starting treatment for their birdshot. Because of this, we decided to publish a ‘question and answer’ section on the website. We hope it will provide you with some much-needed reassurance and answers to many of your questions.
Much of the ‘question and answer’ section may appear to be just common sense, but BUS thought it useful to gather the information together in one place. We also hope it will be helpful to those who are newly diagnosed and who are looking for advice on how to stay well while on immunosuppressive therapy.
Members of the the BUS Standing Advisory Committee and National Birdshot Research Network have been involved in validating this collected information.
If anything is not clear, or if you have other questions or concerns you would like to raise, please do not hesitate to get in touch.
Forthcoming dates for your diary
Following on from the success of last year’s birdshot events, in 2014 BUS has decided to hold:
- two social events which will also incorporate talks about the research that BUS is helping to fund
- a fundraising clay pigeon shoot, and
- we will also be entering a team of walkers for the Fight for Sight Carrots Nightwalk
London social meetings and talks
The London social meetings and talks co-incide with bank holiday weekends. We hope this will make it easier to attend if you don’t live in the south east. The socials will provide an opportunity to socialise with other birdshotters and a chance to learn more about our birdshot uveitis research. The meetings will be held in the delightful environment of a historic Thames barge located in St Katherine’s Dock. As before, a buffet lunch and drink will be provided.
- Saturday 3rd May 2014 London social meeting with Professor Glen Jeffery from UCL and the Institute of Ophthalmology talking about the red light therapy research being carried out at Moorfields Eye Hospital.
- Saturday 23rd August 2014 with Dr Graham Wallace from Birmingham University talking about the development of the birdshot bio-resource centre and national birdshot database.
——–
Fundraisers
- 2nd birdshot shoot and auction on Wednesday 21st May 2014. The venue (Royal Berkshire Shooting Club) and cost of tickets will be identical to last year. If you are interested in taking part as an individual or entering as a team, (4 guns), please register your interest now, because places are already going fast.
- Carrot’s Night Walk – BUS will again be entering a team of walkers for the Fight for Sight Carrots Night walk. The date and details have yet to be confirmed, but it will be on a Friday night in September.
If you are interested in coming along to the socials/talks, or taking part in the fundraising events please do get in touch and make a note in your diaries. Email: info at birdshot.org.uk
Current and emerging birdshot treatment options
Birdshot uveitis: current and emerging treatment options is the title of a recently published paper by Victor Menezo from Institut Catala de Retina, Barcelona, Spain; Department of Ophthalmology, Provincial Hospital Consortium Castellon, Castello, Spain and Simon R J Taylor Faculty of Medicine, Imperial College London, Hammersmith Hospital, London, UK; Royal Surrey County Hospital NHS Foundation Trust, Guildford
(Note: the whole paper can be downloaded as a pdf by following the link above and scrolling to the bottom of the abstract.)
This paper provides a comprehensive summary of current tests and treatments available for Birdshot Uveitis. It clearly makes the important point that, although central visual acuity can be preserved until late in the disease, it is not uncommon for patients to receive inadequate immunosuppressive treatment, leading to a poor long-term outcome in which peripheral retinal damage eventually leads to visual deterioration.
It also states that although “laboratory research continues to investigate the underlying mechanisms of disease, and clinical research is now being driven to improve the phenotyping and monitoring of this condition, it is becoming increasingly important to identify patients at risk of visual loss early so that they can be treated more aggressively with targeted therapies such as the newer biological agents.”
It states that “this approach requires the formation of collaborative groups, as the relative rarity of the condition makes it difficult for one center to accumulate enough patients for worthwhile studies. Nevertheless, results obtained with newer therapies, such as biological agents directed against particular cytokines or cell-surface receptors, demonstrate ever improving control of the inflammation in refractory cases, providing hope that the outlook for visual function in this condition can only improve.”
This paper certainly gives BUS hope. It is gratifying that it clearly endorses our belief about the best way forward, just at the point when we are getting collaborative work off the ground with the National Birdshot Research Network; and the development of the Birdshot bio-resource centre and Birdshot database.
Boston Birdshot Symposium 2013
Last week we were in touch with Alison Justus from MERSI concerning the video footage of the September 2013 Boston Birdshot Symposium which is going to be made available on-line. We are delighted to report that it should be ready early in the New Year and as soon as we have it, we will post the link on the BUS website.
In the meantime, the PDF below shows the posters that were distributed at the US Boston Birdshot conference. They describe the latest published Birdshot research coming out of MERSI.
The posters are:
- Assessment of TH1, TH2 and TH17 Cells in Birdshot Retinochoroidopathy: P Yang and C S Foster
- Combined therapy of cyclosporine A and mycophenolate mofetil for the treatment of birdshot retinochoridopathy: a 12 month follow-up RA Cervantes-Castaneda , LA Gozalez , M Cordero-Coma, T Yilmaz and CS Foster
- Regulatory T-Cells in Peripheral Blood of Patients with Birdshot Retinochoroidopathy: S S Siddique, L Amorese, L Almulki, A M Sueves. C S Foster
- Risk factors associated with relapse of Birdshot Retinochoroidopathy: A E Radwan R Oarujg, U Baheti, M Austin, CS Foster
- Infliximab treatment of patients with Birdshot Retinochoroidopathy:P Artornsombudh, O Gevorgyan, A Payal, SS Siddique, D C Foster
Donna’s infliximab (Remicade®) experience
We really welcome receiving your stories as they can be very helpful especially for newly diagnosed Birdshotters to read. There is nothing like hearing first-hand about stories of treating Birdshot Uveitis successfully to give you hope and help make you realise that there can be life after a Birdshot diagnosis.
Recently I was in touch with Donna, (I met her at the Boston 2013 Birdshot conference) as I wanted her to share her experiences of cyclosporin followed by Remicade®, with a birdshotter from Puerto Rico who had recently written in to BUS and was wanting to communicate with other birdshotters before making her decision about her next treatment option.
We asked Donna if we could share her experience on the BUS website. The answer was yes, so this is her story.
“After my diagnosis four years ago here in Edmonton, Alberta, Canada, I was put on prednisone initially to get a jump on the inflammation. I learned of Dr. Foster in Boston, who apparently sees the most Birdshot patients, and my family insisted I go to see him. As per Dr. Foster’s protocol, I went on his cocktail of mycophenolate moefetil (CellCept®) and cyclosporine and weaned off the prednisone after 3 months. I was on this protocol for 15 months. My Birdshot stopped progressing but none of the symptoms reversed and I continued monthly injections in both eyes to control ongoing macular edema. I had many not-so-nice side effects from the cyclosporine that I was willing to put up with, thinking it was helping, but eventually it attacked my kidneys and I had to stop. I then found out that the amount and severity of the side effects probably meant the drug wasn’t really working for me all that time. Having said that, I met MANY patients at the recent International Birdshot Symposium in Boston who have had success with cyclosporine with NO side effects.
Dr. Foster then recommended I continue with the CellCept® and add Remicade®. While I waited for my insurance to approve the Remicade® my vision plummeted. Seven days after starting Remicade® I experienced a dramatic improvement in my vision! After 14 days I was driving again! My symptoms began reversing, the macular edema stopped (yeah, no more eye injections after two years!) and my vision continued to improve. I do have some permanent damage (from before the Remicade®) that doesn’t really bother me any more now that I am used to it and I am seeing better than I have in four years. And the only side effect I have experienced on Remicade® are some sinus issues that are easily controlled. Remicade® has been a miracle drug for me! Dr. Foster says the current thinking is a minimum of two and preferably three years symptom-free before weaning off all meds.
I don’t know what your health insurance is but it might be a major factor in your decision. cyclosporine and CellCept® are covered by my health care plan. While Remicade® is approved for use in many autoimmune conditions here in Canada, Health Canada has not yet approved it for use in eyes because there have not been studies done with adequate numbers here! It was a hassle getting approval from my husband’s company plan but they finally did approve it. The plan covers 90% of the cost but it is still extremely expensive. I would be inclined to say try the CellCept® and cyclosporine first because so many patients seem to have success with that. The Remicade® is a great back-up.”
Remicade® is given via an infusion in a hospital. It is rarely available in the UK unless other treatments have failed or are not tolerated. A special funding application has to be made. There is also the inconvenience of having to go every 4 or 6 weeks to have the infusion and this generally takes a couple of hours to do.
For further information about a recent research study on infliximab please see: http://www.ncbi.nlm.nih.gov/pubmed/23177362
If you would like to tell others your Birdshot story, please do write it down so we can publish it for our members to read.
Novel treatment for birdshot to be trialled at Moorfields
Can exposure to 670nm infra-red light improve retinal function and structure?
BUS is delighted to announce forthcoming research into a novel treatment for birdshot which is being trialled at Moorfields Eyes Hospital. The Director of Medical Retina and Uveitis, consultant Carlos Pavesio, the lead researcher, will be investigating whether using a particular wavelength of light will reduce inflammation in the eye and improve retinal function and structure in birdshot uveitis.
Please note, that before everybody rushes forward to volunteer, the patients selected from Carlos Pavesio’s clinic will be invited to participate in this research only if they fulfil the necessary criteria, and initially, only 10 to 12 patients will be required. His research team aims to study the effects of 670nm infra-red light treatment on patients with ‘grumbling’ birdshot disease, with their signs of inflammation continuing to be treated with medication throughout the trial.
Funding for the project
Half of the money for this research proposal was raised by birdshot walkers on the 2012 Fight for Sight ‘Carrots Night Walk’. We are collaborating with Fight for Sight, the main UK charity dedicated to funding eye research to prevent sight loss and treat eye disease, who have matched our fundraising to help pay for this novel piece of research via their jointly funded Small Grant Awards programme.
Targeted treatment
Birdshot is an eye disease with no known involvement of other parts of the body. Because of this, effective treatment directed just to the eyes would be a big step forward.
670nm infra-red is a part of the natural light spectrum. The energy levels and wavelength of the 670nm light used in the study are lower than those of indirect light on a spring morning. This light wavelength is also emitted from conventional light bulbs, but it is absent from strip lights and also absent from all the new low energy eco-friendly domestic light bulbs.
Study patients will receive 670nm light treatment from a specially-designed light. Treatments will be given on weekdays for two weeks for a total of ten treatments. Each light treatment lasts one minute. Only one eye per patient will be treated but the other eye will be observed as a ‘control’ for comparison.
In active birdshot uveitis, there is a measurable delay in response to the 30Hz ERG flicker test stimulus, so before the first light treatment for each patient in the trial, a baseline comprehensive ERG will be done. During the trial, patients will be given further ERGs before and after each light treatment, and these ERGs will be performed with a hand-held ERG device (RETeval) where the electrodes are placed on the skin under the eye. These pre- and post-treatment ERGs will check if the light treatment has improved the ERG response.
It will take no more than two minutes to do the study ERG tests, and the eyes do not have to be dilated. The ERG results will be immediately available, so the clinicians will quickly know if there are any improvements in the eyes.
Depending on the results of this small trial, the researchers aim to run a larger study over a longer period of time to assess the usefulness of this special light therapy as a treatment option for birdshot.
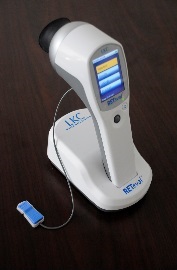 See the Youtube video of the RETeval ERG device by clicking this link
See the Youtube video of the RETeval ERG device by clicking this link
This project combines the skills of the medical retina department at Moorfields Eye Hospital with the experience of using this therapy in the laboratory environment of the Institute of Ophthalmology. Both places are world leaders in addressing ophthalmic problems.
Here’s to success with this study. We will be very interested to hear how the project progresses.
Birmingham gets birdshot registry and bio-bank resource centre underway
BUS is delighted to announce that two exciting research projects are about to commence.
1) the development of a National Birdshot Bio-Resource Centre and;
2) the development of a National Birdshot Uveitis Registry.
These two projects are coming to fruition thanks to the hard work of Birdshot Uveitis Society supporters who have helped raise the money and also thanks to the imagination, ingenuity and resourcefulness of clinicians and research scientists who are involved in the National Birdshot Research Network.
The Bio-resource Centre project is being jointly funded by BUS and Fight for Sight, the main UK charity dedicated to funding eye research to prevent sight loss and treat eye disease, via a jointly funded Small Grant Award of £15,000. 50% of the costs has been raised by Birdshotters who took part in the 2012 Carrots Nightwalk, a six and 15 mile charity walk, with matched funding from Fight for Sight.
The development costs of the National Birdshot Uveitis registry is being funded by BUS with money our supporters have donated over the last year and donations from the BUS 2013 Shoot which is our major fundraiser. This award will be administered by Fight for Sight.
Thank you so much to everyone for your time and generous support. We wouldn’t have got this far without it!
World’s first birdshot uveitis biobank project
This research proposal is one you may have previously seen mentioned here: establishing in the UK the world’s first birdshot uveitis biobank. Biobanks collect and store donations of anonymised patient samples of blood, urine and other tissues. Approved researchers can then use these materials, feeding back their research data to the biobank. This adds to the original information held about the samples, which increases their usefulness to future researchers.
Rather than set up an independent birdshot biobank, it makes economic sense to use an existing biobank. The established Infectious Diseases Biobank at King’s College Hospital, London, has expanded to include collections of samples from patients with inflammatory conditions. This means that the Birmingham-based researchers’ proposal to set up the birdshot biobank within the biobank at King’s College Hospital fits in well with King’s existing work.
The project will start by recruiting a small number of birdshot patients from St Thomas’ Hospital, London, Moorfields Eye Hospital, London, and the Birmingham and Midlands Eye Centre, to donate samples to the biobank.
Clinical data from the National Birdshot Uveitis Registry (see below) would accompany the biobank samples. The combined resources of the National Birdshot Uveitis Registry and the National Birdshot Biobank would enable a great variety of statistically-valid and useful studies to be performed on large numbers of birdshot patient samples.
It is hoped that the birdshot biobank will eventually provide the opportunity for all UK birdshotters to contribute to research by donating their samples. Our involvement will provide the keys to help researchers start to unlock some of the mysteries of birdshot and to improve its treatment.
Developing a UK-wide National Birdshot Uveitis Registry
To be statistically significant, medical studies need to be done on large numbers of patients. Because birdshot is a rare disease, it has been difficult up to now to recruit enough patients to do useful research. Although large studies of up to 80 birdshot patients have been carried out in several European centres, similar scale studies have not been conducted in the UK, principally because birdshot patient information is not held in one place. Creating a national registry database of UK birdshot patients would provide a resource of information on birdshot which would have many potential uses.
The proposed National Birdshot Uveitis Registry (NBUR) will collect and hold clinical data on birdshot patients centrally, including test results such as angiograms and OCT scan images. From the initial pilot study involving data from a few patients at University Hospitals Birmingham and the Birmingham and Midland Eye Centre, the eventual aim is for all UK birdshot patients to be invited to register with the NBUR.
The first step in the proposed study will be to develop a patient details database at University Hospitals Birmingham, test it and perform some data analysis. The database will then be tried out with data from a small number of Birmingham birdshot patients, then modified as necessary, and then piloted at National Birdshot Research Network partner sites in the UK.
One use of the registry data will be to accompany the samples sent to the UK birdshot research biobank (see above). Other proposed uses for the data include identifying patients suitable for clinical trials, collecting drug safety data, especially for newer treatments, and eventually being able to identify ‘best practice’ and promote better care by enabling treatment centres to compare the progress of their patients within the context of a national picture.
University Hospitals Birmingham already develops and operates national databases through their established Rare Disease Service, so this experience will be invaluable in setting up the National Birdshot Uveitis Registry.